Animated Chemical Bonding
Students create animations to demonstrate their understanding of a chemical process.
App: Frames™

Task
Now that you have studied the different types of chemical bonds (ionic, covalent, and metallic), create a clay animation model that will help the students in next year’s course understand how valence shell electrons affect the types of bonds that different elements make.
Engage
This project is a great summative assessment after you have introduced and explained the various types of chemical bonding.
Chemistry deals with concepts at a molecular or atomic level, making students think abstractly or conceptually. In chemistry courses, you complete experiments and create concrete models that demonstrate what is happening at a molecular level to help students understand these concepts. Having them create clay animations to demonstrate processes and models also helps make these concepts tangible.
This project is a culminating activity to reinforce and remind students of concepts you have covered. So, before they begin working on a clay animation, cover, and UNcover, important chemistry concepts through reading, models, lab work, and discussions.
Before creating animations that demonstrate chemical bonding, students must be familiar with how chemical bonds form. Share examples of the various types of bonds: ionic, covalent, and metallic. Ionic bonding involves the transfer of electrons, covalent bonding involves sharing of electrons, and metallic bonding can be considered a combination of both.
Chemical bonds form to lower the energy of the system. Each component of a system becomes more stable by forming a bond. A full valence shell equals stability.
Students should be able to explain how the valence shell of electrons affects the type of bonding between elements and how compounds bond because of electrostatic charge.
Create
Have students form into small teams. Assign each team a type of bond to model.
Have each team develop their own definition for their bond. Have them brainstorm at least five examples of elements or compounds that form this type of bond.
Have students create a visual model of the bond. The model should also include a description of the bond and a justification for the elements they chose to demonstrate the bond. Evaluate the model for accuracy. Have students make necessary changes.
Once the model is complete, have students use a storyboard to map out how their animation will demonstrate the chemical bond. The team should write and develop a narrative script to explain how the bond is made in this example, and share other examples of where this type of bond can be found.
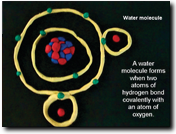
Have each team create a clay animation simulation of their chemical bond. The animations should contain models of each atom/element showing protons, neutrons, and electrons and their charges. Models should also demonstrate how these charges and structures contribute to the type of bond this element makes.
Share
Have the students share their clay animations with the rest of the class. Use this as a review before an exam. You might also want to share them with a local access television station and other chemistry classes in your area.
Assessment
The introductory activities, including discussions, reading, and lab work, will build a foundation of knowledge appropriate for students to create the animation.
As the teams work, be sure to evaluate their initial model. Their description of how the bond works will provide insight into their thinking. Ask questions to test their knowledge about the bond. Be sure to ask why they chose the elements or molecules used in the model.
The storyboard is a clue into how well students can verbalize and visualize the chemical bond for someone else. The animation is a culmination of their model, storyboard, planning, and teamwork. Students’ ability to answer peer questions after showing their animation will indicate comfort and fluency with the concept.
Resources
Winter, Mark J. Chemical Bonding. (Oxford Chemistry Primers, No 15). ISBN: 0198556942
Periodic Table of the Elements
Standards
NSEA–National Science Education
As a result of their activities in grades 9–12, all students should develop an understanding of the structure of atoms, the structure and properties of matter, and chemical reactions. (Physical Science–CONTENT STANDARD B)
ISTE NETS for Students 2016:
6. Creative Communicator
Students communicate clearly and express themselves creatively for a variety of purposes using the platforms, tools, styles, formats and digital media appropriate to their goals. Students:
a. choose the appropriate platforms and tools for meeting the desired objectives of their creation or communication.
b. create original works or responsibly repurpose or remix digital resources into new creations.
c. communicate complex ideas clearly and effectively by creating or using a variety of digital objects such as visualizations, models or simulations.
d. publish or present content that customizes the message and medium for their intended audiences.